The "bath-tub ring" model of Mn ore genesis
Manganese ore deposits, all dating from the Early Oligocene, form an arc around the north side of the Black Sea, stretching from Turkey through Bulgaria, the Ukraine, Russia and Georgia and finally into Kazakhstan. They constitute the third and youngest great episode of Mn deposition.
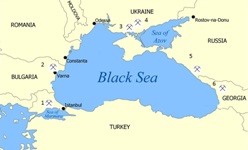 Black Sea deposits (click for larger image). Not shown is Mangyshlak in Kazakhstan.
Black Sea deposits (click for larger image). Not shown is Mangyshlak in Kazakhstan.
Table of average ore compositions
These and most other large Mn deposits likely formed around the margins of euxinic basins (those with free H2S in deep water). Such basins -- exemplified by the modern Black Sea -- have a thin zone of oxygen-containing water overlying a large reservoir of oxygen-free, sulfidic water. Elements like Mn that are soluble under reducing, sulfidic conditions but insoluble under oxidizing conditions precipitate along the interface between these water masses. Generally the Mn solids simply sink back into the sulfidic water and redissolve, but along basin margins, where the transition impinges on the bottom, Mn oxide accumulates in the bottom sediment where it reacts with organic matter to form Mn carbonate (Force and Cannon,1988; Okita, 1987; Okita et al. 1988; Maynard et al., 1990; Okita 1992, and Okita and Shanks, 1992).
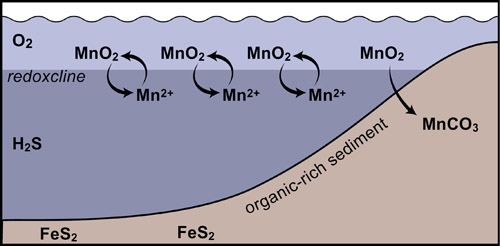
The overall process can be represented schematically by the reaction
2MnO2 + CH2O + HCO3- → 2MnCO3 + H2O + OH- .
1/2 the C incorporated in the MnCO3 comes from organic matter, which has an isotopic composition of ~ -27 permil, while the remaining 1/2 comes from oceanic HCO3 with an isotopic composition close to 0 permil.
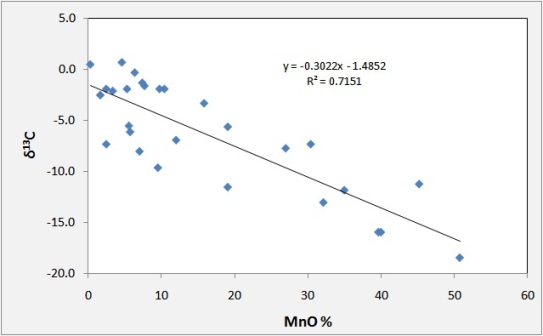
In the ideal case, the resulting Mn deposit will have a good correlation between Mn grade and d13C with high grade samples reaching -15 permil, as seen here for Usa, in the Urals region of Russia.